Abstract
Introduction: Immune thrombocytopenia (ITP) is an autoimmune disease characterized by a low platelet count (≤100x109/L) due to platelet destruction and insufficient platelet production. There is a wide variation in the presentation of the disease, the clinical course and the response to therapeutic treatments.
It has been proposed that certain autoimmune diseases might be caused by changes in glycosylation of cell surface proteins which induce the immune system to recognize cells as "non-self". The aim of this work was to evaluate whether glycosylation might be involved in ITP ethiopathogenesis. So, in this preliminary study we aimed to evaluate the glycosylation pattern of platelets from ITP patients, its relationship with platelet features and with the response to treatments.
Methods: Seventy eight ITP patients were recruited: 32 without treatment (UT-ITP) for at least six months, 35 responders to thrombopoietin receptor agonists (TPO-RA, 62.8% with eltrombopag, 27.2 % with romiplostim), and 11 refractory (r-ITP). Eighty one healthy controls were also included.
Human peripheral blood samples were collected in 3.8% sodium citrate.
Platelet activation was determined by flow cytometry (FCM) through binding of FITC-PAC1 (a mAb that recognizes the activated conformation of fibrinogen receptor) after activation with 100 mM thrombin receptor-activating peptide 6 (TRAP, Bachem, Switzerland). Apoptosis was evaluated by measurement of active caspase-3, -8 or -9 by FCM with a specific kit (Millipore, Madrid, Spain).
Platelet surface exposure of lectins was analyzed with 1 µg/ml FITC-conjugated Wheat germ agglutinin lectin (WGA) or 1 µg/ml FITC-conjugated Erythrina cristagalli lectin (ECA). WGA binds to sialic acid and N-acetylglucosaminyl residues and ECA is a galactose specific legume lectin.
Glycoprotein derived glycans from a healthy control and from a r-ITP patient were characterized by MALDI-MS based glycomic approaches. N-linked glycans were released from platelet glycoproteins by PNGase F digestion whilst O-linked glycans were released by reductive elimination. Both pools of glycans were permethylated prior to MALDI-MS.
Comparisons of quantitative variables were made with SPSS.22 software.
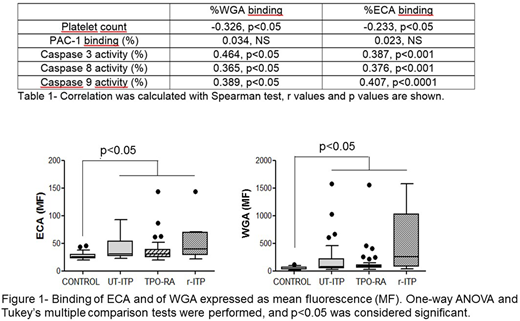
Results: Platelet count (x109/L) was 269±55 in controls, 141±126 in UT-ITP, 111±85 in TPO-RA and 8±5 in r-ITP. ITP patients with the lowest platelet count showed the most pronounced binding of WGA and ECA (Table 1). Moreover, binding of these lectins showed a mild positive correlation with caspase activities (Table 1). No relationship was observed between platelet ability to be activated and glycosylation features.
When lectins' binding was studied stratifying patients according to their response to therapeutic treatments we observed that platelets from all ITP patients bound more ECA and WGA (Figure 1). Despite no significant differences were observed between ITP groups, r-ITP seemed to bind more WGA.
To increase our knowledge about protein glycosylation of platelet proteins in ITP patients we performed glycomic analyses of platelets from one healthy control and from one r-ITP patient. It was demonstrated that bi-, tri- and tetra-antennary complex type N-glycans are the dominant class of N-glycans with also some high mannose structures. Analysis of sialylated N-glycan, via digestion with sialidase, revealed lower levels of sialylation in r-ITP patient platelets compared to healthy control platelets. Platelet O-glycan data revealed the presence of mucin-type core-1 and core-2 structures.
Conclusion: Glycomic analysis demonstrates that human platelet glycoproteins are modified with a complex array of both N- and O-linked glycans. Preliminary data indicates that there could be changes in glycosylation associated with immune thrombocytopenia. These changes in platelet protein glycosylation might be involved in an enhancement of apoptosis in platelets from ITP patients.
Work supported by grant from FIS-FEDER PI15/01457. NB holds a Miguel Servet II (FIS-FEDER CP14/00024).
Álvarez-Roman:Shire: Consultancy; NovoNordisk: Consultancy; SOBI: Consultancy. Jimenez-Yuste:Bayer: Consultancy, Research Funding; Sobi: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Grifols: Consultancy, Research Funding; Octapharma: Consultancy, Research Funding; CSL Behring: Consultancy; NovoNordisk: Consultancy, Research Funding; Shire: Consultancy, Research Funding. Butta:FIS-Fondos FEDER: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal